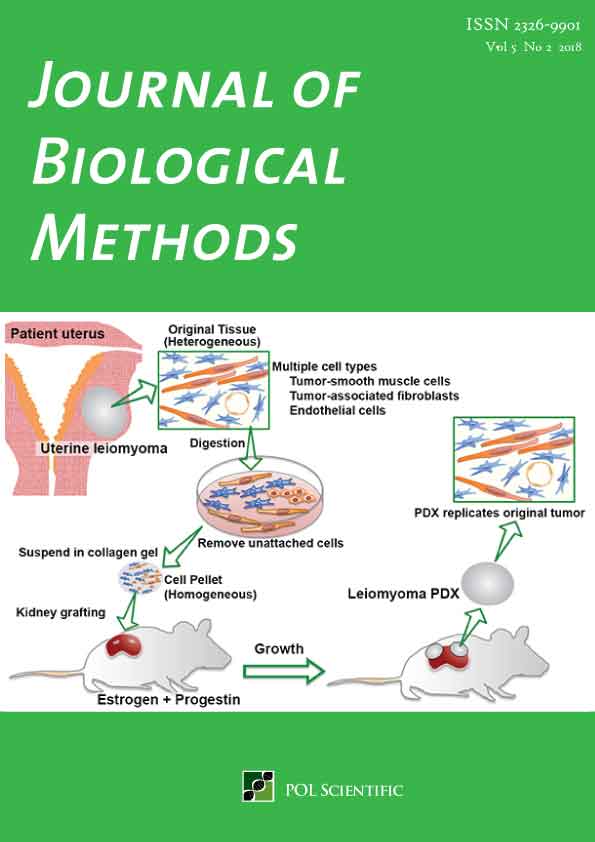
Patient-derived xenograft model for uterine leiomyoma by sub-renal capsule grafting
Main Article Content
Keywords
estradiol, fibroids, myometrium, NSG mouse, patient-derived xenografts, preclinical model, progesterone
Abstract
Uterine leiomyoma (UL) or fibroid is a benign smooth muscle tumor of the myometrium with a lifetime incidence of approximately 70%. ULs often require medical intervention due to severe symptoms such as heavy menstrual bleeding and abdominal pain. Although the most common and effective management of ULs is surgical removal, the invasive surgical procedure imposes physical and psychological burdens on the patients. Moreover, the economic burden of UL on health care system is enormous due to the high cost of surgeries. Thus, therapeutic options with long-term efficacy to replace surgical management are urgently needed. For the development of such medical options, reliable preclinical research models are imperative. Ex vivo culture of UL cells has been the primary research model for decades. However, recent studies demonstrated that primary cell culture is not a suitable model for UL research, as primary cultures of ULs mostly consist of non-tumor fibroblasts. Here we describe the protocol for patient-derived xenograft of UL, which faithfully replicates the phenotypes of human UL in situ.
Downloads
Download data is not yet available.
Metrics
Metrics Loading ...
References
Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. American journal of obstetrics and gynecology. 2003;188(1):100-7. PubMed PMID: 12548202.
2. Hehenkamp WJ, Volkers NA, Bartholomeus W, de Blok S, Birnie E, Reekers JA, et al. Sexuality and body image after uterine artery embolization and hysterectomy in the treatment of uterine fibroids: a randomized comparison. Cardiovasc Intervent Radiol. 2007;30(5):866-75. doi: 10.1007/s00270-007-9121-7. PubMed PMID: 17671809; PubMed Central PMCID: PMC2039794.
3. Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. American journal of obstetrics and gynecology. 2012;206(3):211 e1-9. doi: 10.1016/j.ajog.2011.12.002. PubMed PMID: 22244472; PubMed Central PMCID: PMC3292655.
4. Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433-42. Epub 2010/04/09. doi: 10.1210/en.2009-1225. PubMed PMID: 20375184; PubMed Central PMCID: PMC2875812.
5. Murji A, Whitaker L, Chow TL, Sobel ML. Selective progesterone receptor modulators (SPRMs) for uterine fibroids. Cochrane database of systematic reviews (Online). 2017;4:CD010770. doi: 10.1002/14651858.CD010770.pub2. PubMed PMID: 28444736.
6. Mäkinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science (New York, NY. 2011;334(6053):252-5. Epub 2011/08/27. doi: 10.1126/science.1208930. PubMed PMID: 21868628.
7. Mehine M, Kaasinen E, Heinonen HR, Mäkinen N, Kampjarvi K, Sarvilinna N, et al. Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(5):1315-20. doi: 10.1073/pnas.1518752113. PubMed PMID: 26787895; PubMed Central PMCID: PMC4747776.
8. Markowski DN, Bartnitzke S, Loning T, Drieschner N, Helmke BM, Bullerdiek J. MED12 mutations in uterine fibroids--their relationship to cytogenetic subgroups. International journal of cancer. 2012;131(7):1528-36. Epub 2012/01/10. doi: 10.1002/ijc.27424. PubMed PMID: 22223266.
9. Bertsch E, Qiang W, Zhang Q, Espona-Fiedler M, Druschitz S, Liu Y, et al. MED12 and HMGA2 mutations: two independent genetic events in uterine leiomyoma and leiomyosarcoma. Mod Pathol. 2014;27(8):1144-53. Epub 2014/01/07. doi: 10.1038/modpathol.2013.243. PubMed PMID: 24390224; PubMed Central PMCID: PMC4081525.
10. Wu X, Serna VA, Thomas J, Qiang W, Blumenfeld ML, Kurita T. Subtype-specific tumor-associated fibroblasts contribute to the pathogenesis of uterine leiomyoma. Cancer Res. 2017. doi: 10.1158/0008-5472.CAN-17-1744. PubMed PMID: 29055020.
11. Markowski DN, Tadayyon M, Bartnitzke S, Belge G, Maria Helmke B, Bullerdiek J. Cell cultures in uterine leiomyomas: rapid disappearance of cells carrying MED12 mutations. Genes, chromosomes & cancer. 2014;53(4):317-23. Epub 2014/01/22. doi: 10.1002/gcc.22142. PubMed PMID: 24446130.
12. Bloch J, Holzmann C, Koczan D, Helmke BM, Bullerdiek J. Factors affecting the loss of MED12-mutated leiomyoma cells during in vitro growth. Oncotarget. 2017;8(21):34762-72. doi: 10.18632/oncotarget.16711. PubMed PMID: 28410233; PubMed Central PMCID: PMC5471009.
13. Wang Y, Wang JX, Xue H, Lin D, Dong X, Gout PW, et al. Subrenal capsule grafting technology in human cancer modeling and translational cancer research. Differentiation; research in biological diversity. 2016;91(4-5):15-9. doi: 10.1016/j.diff.2015.10.012. PubMed PMID: 26547391.
14. Wang G, Ishikawa H, Sone K, Kobayashi T, Kim JJ, Kurita T, et al. Nonobese diabetic/severe combined immunodeficient murine xenograft model for human uterine leiomyoma. Fertility and sterility. 2014;101(5):1485-92. doi: 10.1016/j.fertnstert.2014.01.054. PubMed PMID: 24636398.
15. Qiang W, Liu Z, Serna VA, Druschitz SA, Liu Y, Espona-Fiedler M, et al. Down-Regulation of miR-29b Is Essential for Pathogenesis of Uterine Leiomyoma. Endocrinology. 2014;155(3):663-9. Epub 2014/01/16. doi: 10.1210/en.2013-1763. PubMed PMID: 24424054; PubMed Central PMCID: PMC3929741.
16. Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P, Stricker R. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med. 2006;44(7):883-7. doi: 10.1515/CCLM.2006.160. PubMed PMID: 16776638.
17. Cunha GR, Baskin L. Use of sub-renal capsule transplantation in developmental biology. Differentiation; research in biological diversity. 2016;91(4-5):4-9. doi: 10.1016/j.diff.2015.10.007. PubMed PMID: 26639079; PubMed Central PMCID: PMC4884165.
18. Kurita T, Medina R, Schabel AB, Young P, Gama P, Parekh TV, et al. The activation function-1 domain of estrogen receptor alpha in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation; research in biological diversity. 2005;73(6):313-22. Epub 2005/09/06. doi: 10.1111/j.1432-0436.2005.00033.x. PubMed PMID: 16138832.
19. Terakawa J, Rocchi A, Serna VA, Bottinger EP, Graff JM, Kurita T. FGFR2IIIb-MAPK Activity Is Required for Epithelial Cell Fate Decision in the Lower Mullerian Duct. Molecular endocrinology (Baltimore, Md. 2016;30(7):783-95. doi: 10.1210/me.2016-1027. PubMed PMID: 27164167; PubMed Central PMCID: PMC4926232.
20. Cunha GR, Vanderslice KD. Identification in histological sections of species origin of cells from mouse, rat and human. Stain Technol. 1984;59(1):7-12. PubMed PMID: 6206625.
2. Hehenkamp WJ, Volkers NA, Bartholomeus W, de Blok S, Birnie E, Reekers JA, et al. Sexuality and body image after uterine artery embolization and hysterectomy in the treatment of uterine fibroids: a randomized comparison. Cardiovasc Intervent Radiol. 2007;30(5):866-75. doi: 10.1007/s00270-007-9121-7. PubMed PMID: 17671809; PubMed Central PMCID: PMC2039794.
3. Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. American journal of obstetrics and gynecology. 2012;206(3):211 e1-9. doi: 10.1016/j.ajog.2011.12.002. PubMed PMID: 22244472; PubMed Central PMCID: PMC3292655.
4. Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433-42. Epub 2010/04/09. doi: 10.1210/en.2009-1225. PubMed PMID: 20375184; PubMed Central PMCID: PMC2875812.
5. Murji A, Whitaker L, Chow TL, Sobel ML. Selective progesterone receptor modulators (SPRMs) for uterine fibroids. Cochrane database of systematic reviews (Online). 2017;4:CD010770. doi: 10.1002/14651858.CD010770.pub2. PubMed PMID: 28444736.
6. Mäkinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science (New York, NY. 2011;334(6053):252-5. Epub 2011/08/27. doi: 10.1126/science.1208930. PubMed PMID: 21868628.
7. Mehine M, Kaasinen E, Heinonen HR, Mäkinen N, Kampjarvi K, Sarvilinna N, et al. Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(5):1315-20. doi: 10.1073/pnas.1518752113. PubMed PMID: 26787895; PubMed Central PMCID: PMC4747776.
8. Markowski DN, Bartnitzke S, Loning T, Drieschner N, Helmke BM, Bullerdiek J. MED12 mutations in uterine fibroids--their relationship to cytogenetic subgroups. International journal of cancer. 2012;131(7):1528-36. Epub 2012/01/10. doi: 10.1002/ijc.27424. PubMed PMID: 22223266.
9. Bertsch E, Qiang W, Zhang Q, Espona-Fiedler M, Druschitz S, Liu Y, et al. MED12 and HMGA2 mutations: two independent genetic events in uterine leiomyoma and leiomyosarcoma. Mod Pathol. 2014;27(8):1144-53. Epub 2014/01/07. doi: 10.1038/modpathol.2013.243. PubMed PMID: 24390224; PubMed Central PMCID: PMC4081525.
10. Wu X, Serna VA, Thomas J, Qiang W, Blumenfeld ML, Kurita T. Subtype-specific tumor-associated fibroblasts contribute to the pathogenesis of uterine leiomyoma. Cancer Res. 2017. doi: 10.1158/0008-5472.CAN-17-1744. PubMed PMID: 29055020.
11. Markowski DN, Tadayyon M, Bartnitzke S, Belge G, Maria Helmke B, Bullerdiek J. Cell cultures in uterine leiomyomas: rapid disappearance of cells carrying MED12 mutations. Genes, chromosomes & cancer. 2014;53(4):317-23. Epub 2014/01/22. doi: 10.1002/gcc.22142. PubMed PMID: 24446130.
12. Bloch J, Holzmann C, Koczan D, Helmke BM, Bullerdiek J. Factors affecting the loss of MED12-mutated leiomyoma cells during in vitro growth. Oncotarget. 2017;8(21):34762-72. doi: 10.18632/oncotarget.16711. PubMed PMID: 28410233; PubMed Central PMCID: PMC5471009.
13. Wang Y, Wang JX, Xue H, Lin D, Dong X, Gout PW, et al. Subrenal capsule grafting technology in human cancer modeling and translational cancer research. Differentiation; research in biological diversity. 2016;91(4-5):15-9. doi: 10.1016/j.diff.2015.10.012. PubMed PMID: 26547391.
14. Wang G, Ishikawa H, Sone K, Kobayashi T, Kim JJ, Kurita T, et al. Nonobese diabetic/severe combined immunodeficient murine xenograft model for human uterine leiomyoma. Fertility and sterility. 2014;101(5):1485-92. doi: 10.1016/j.fertnstert.2014.01.054. PubMed PMID: 24636398.
15. Qiang W, Liu Z, Serna VA, Druschitz SA, Liu Y, Espona-Fiedler M, et al. Down-Regulation of miR-29b Is Essential for Pathogenesis of Uterine Leiomyoma. Endocrinology. 2014;155(3):663-9. Epub 2014/01/16. doi: 10.1210/en.2013-1763. PubMed PMID: 24424054; PubMed Central PMCID: PMC3929741.
16. Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P, Stricker R. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med. 2006;44(7):883-7. doi: 10.1515/CCLM.2006.160. PubMed PMID: 16776638.
17. Cunha GR, Baskin L. Use of sub-renal capsule transplantation in developmental biology. Differentiation; research in biological diversity. 2016;91(4-5):4-9. doi: 10.1016/j.diff.2015.10.007. PubMed PMID: 26639079; PubMed Central PMCID: PMC4884165.
18. Kurita T, Medina R, Schabel AB, Young P, Gama P, Parekh TV, et al. The activation function-1 domain of estrogen receptor alpha in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation; research in biological diversity. 2005;73(6):313-22. Epub 2005/09/06. doi: 10.1111/j.1432-0436.2005.00033.x. PubMed PMID: 16138832.
19. Terakawa J, Rocchi A, Serna VA, Bottinger EP, Graff JM, Kurita T. FGFR2IIIb-MAPK Activity Is Required for Epithelial Cell Fate Decision in the Lower Mullerian Duct. Molecular endocrinology (Baltimore, Md. 2016;30(7):783-95. doi: 10.1210/me.2016-1027. PubMed PMID: 27164167; PubMed Central PMCID: PMC4926232.
20. Cunha GR, Vanderslice KD. Identification in histological sections of species origin of cells from mouse, rat and human. Stain Technol. 1984;59(1):7-12. PubMed PMID: 6206625.